Accurate Spectroscopic Characterization and Formation Pathways of Ethane 1, 1 diol
A Potential Interstellar Molecule
DOI:
https://doi.org/10.62050/ljsir2025.v3n1.393Keywords:
1,1 ethanediol, spectroscopic constants, formation pathways, astrochemistryAbstract
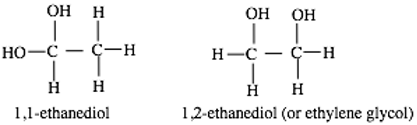
With exceptions, the correlation between relative energies of isomers and their relative abundances in the interstellar medium (ISM) holds to an extent. Among the C2H6O2 isomers, ethylene glycol is the only known interstellar isomer but there is no report regarding the astronomical searches for ethane 1,1-ethanediol (the most stable isomer of the group) due to lack of spectroscopic and other parameters that would have warranted the search. In this article, the most energetically stable conformer of 1,1-ethanediol was investigated, its spectroscopic, and other parameters are obtained from high level ab initio quantum chemical methods. Accurate spectroscopic parameters are obtained at the CCSD(T) level. The proposed formation route of ethane 1,1-diol has a surmountable barrier considering the nature/abundance of the participating species and the energy sources in ISM. The astrophysical implications of these results are discussed and the astronomical searches of ethene 1, 1 diol are proposed.
Downloads
References
Bennett, C. J., & Kaiser, R. I. (2007). The formation of acetic acid (CH3COOH) in interstellar ice analogs. The Astrophysical Journal, 660(2), 1289–1295. https://doi.org/10.1086/513267
Charnley, S. B. (2001). In F. Giovannelli (Ed.), The bridge between the Big Bang and biology (Special Vol., p. 139). Consiglio Naz. Ric.
Davies, J. A., Besley, N. A., Yang, S., & Ellis, A. M. (2019). Probing elusive cations: Infrared spectroscopy of protonated acetic acid. The Journal of Physical Chemistry Letters, 10(9), 2108–2112. https://doi.org/10.1021/acs.jpclett.9b00767
Deng, C., & Zhang, K. (2021). Thermal conductivity of 1,2-ethanediol and 1,2-propanediol binary aqueous solutions at temperature from 253 K to 373 K. International Journal of Thermophysics, 42(6). https://doi.org/10.1007/s10765-021-02837-6
Enrique-Romero, J., Rimola, A., Ceccarelli, C., & Balucani, N. (2016). The (impossible?) formation of acetaldehyde on the grain surfaces: Insights from quantum chemical calculations. Monthly Notices of the Royal Astronomical Society: Letters, 459(1), L6–L10. https://doi.org/10.1093/mnrasl/slw031
Etim, E. E, and E. Arunan (2015). Rotational Spectroscopy and Interstellar Molecules. Planex Newsletter, 5 (2): 16-21. Invited mini-review article.
Etim, E. E, and E. Arunan (2016). Interstellar Isomeric Species: Energy, Stability and Abundance Relationship. European Physical Journal Plus, 131:448. https://doi.org/10.1140/epjp/i2016-16448-0
Etim, E. (2023). Benchmark studies on the isomerization enthalpies for interstellar molecular species. Journal of the Nigerian Society of Physical Sciences, 5(27). https://doi.org/10.46481/jnsps.2023.527
Etim, E. E and E. Arunan (2017a). Accurate Rotational Constants for linear Interstellar Carbon Chains: Achieving Experimental Accuracy. Astrophysics and Space Science, 362, 4. https://doi.org/10.1007/s10509-016-2979-6
Etim, E. E and E. Arunan (2017b). Partition Function and Astronomical Observation of Interstellar Isomers: Is there a link? Advances in Space Research, 59(4)1161-1171.
Etim, E. E, Prsanta Gorai, Ankan Das, Sandip Charabati and E. Arunan (2016). Systematic Theoretical Study on the Interstellar Carbon Chain Molecules. The Astrophysical Journal, 832,144. https://doi.org/10.3847/0004-637X/832/2/144
Etim, E. E., & Arunan, E. (2016). Interstellar isomeric species: Energy, stability and abundance relationship. European Physical Journal Plus, 131(12). https://doi.org/10.1140/epjp/i2016-16448-0
Etim, E. E., Gorai, P., Das,A., Chakrabarti, S. K and Arunan, E. 2018b. Interstellar Hydrogen Bonding. Advances in Space Research, 61(11): 2870-2880, https://doi.org/10.1016/j.asr.2018.03.003.
Etim, E. E., Prsanta Gorai, Ankan Das, and E. Arunan (2017).C5H9N Isomers: Pointers to Possible Branched Chain Interstellar Molecules. European Physical Journal D, 71:86.
Etim, E. E., Prsanta Gorai, Ankan Das, and E. Arunan (2018a). Theoretical investigation of interstellar C–C–O and C–O–C bonding backbone molecules. Astrophysics and Space Science, 363:6. https://doi.org/10.1007/s10509-017-3226-5
Franco, B., Blumenstock, T., Cho, C., Clarisse, L., Clerbaux, C., Coheur, P.-F., & Taraborrelli, D. (2021). Ubiquitous atmospheric production of organic acids mediated by cloud droplets. Nature, 593(7858), 233–237. https://doi.org/10.1038/s41586-021-03462-x
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Petersson, G. A., Nakatsuji, H., Li, X., Caricato, M., Marenich, A., Bloino, J., Janesko, B. G., Gomperts, R., Mennucci, B., Hratchian, H. P., Ortiz, J. V., Izmaylov, A. F., ... Fox, D. J. (2016). Gaussian 09 (Revision A.02) [Computer software]. Gaussian, Inc.
Harding, M.E., Metzroth, J. T., Gauss, and Auer, A.A. (2008). J. Chem. Theor. Comp. 4, 64
Herbst, E. (1995). Chemistry in the interstellar medium. Annual Review of Physical Chemistry, 46(1), 27–54. https://doi.org/10.1146/annurev.pc.46.100195.000331
Hollis, J. M., Lovas, F. J., Jewell, P. R., & Coudert, L. H. (2002). Interstellar Antifreeze: Ethylene Glycol. The Astrophysical Journal, 571(1), L59–L62. https://doi.org/10.1086/341148
Hollis, J. M., Lovas, F. J., Jewell, P. R., & Coudert, L. H. (2002). Interstellar Antifreeze: Ethylene Glycol. The Astrophysical Journal, 571(1), L59–L62. http://dx.doi.org/10.1016/j.asr.2016.11.021
Inostroza-Pino, N., Emmanuel Godwin, O., Mardones, D., & Ge, J. (2024). Formation pathways of formic acid (HCOOH) in regions with methanol ices. Astronomy & Astrophysics, 688, A140. https://doi.org/10.1051/0004-6361/202346642
Manna, A., Pal, S., & Viti, S. (2024). Detection of antifreeze molecule ethylene glycol in the hot molecular core G358.93–0.03 MM1. Monthly Notices of the Royal Astronomical Society, 533(1), 1143–1155. https://doi.org/10.1093/mnras/stae1864
Mehringer, D. M., Snyder, L. E., Miao, Y., & Lovas, F. J. (1997). Detection and confirmation of interstellar acetic acid. The Astrophysical Journal, 480(1), L71.
Nagy, P. I., Dunn, W. J., III, Alagona, G., & Ghio, C. (1991). Theoretical calculations on 1,2-ethanediol. Gauche-trans equilibrium in gas-phase and aqueous solution. Journal of the American Chemical Society, 113(18), 6719–6729. https://doi.org/10.1021/ja00018a002
Naik, V., Voulgarakis, A., Fiore, A. M., Horowitz, L. W., Lamarque, J.-F., Lin, M., Prather, M. J., Young, P. J., Bergmann, D., Cameron-Smith, P. J., Cionni, I., Collins, W. J., Dalsøren, S. B., Doherty, R., Eyring, V., Faluvegi, G., Folberth, G. A., Josse, B., Lee, Y. H., Mackenzie, I. A., Nagashima, T., van Noije, T. P. C., Plummer, D. A., Righi, M., Rumbold, S. T., Skeie, R., Shindell, D. T., Stevenson, D. S., Strode, S., Sudo, K., Szopa, S., & Zeng, G. (2013). Preindustrial to present-day changes in tropospheric hydroxyl radical and methane lifetime from the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP). Atmospheric Chemistry and Physics, 13(10), 5277–5298. https://doi.org/10.5194/acp-13-5277-2013
National Aeronautics and Space Administration, Science Mission Directorate. (2010). Infrared Waves. Retrieved [insert date - e.g. August 10, 2016], from NASA Science website: http://science.nasa.gov/ems/07_infraredwaves
National Center for Biotechnology Information. (n.d.). PubChem compound summary for CID 7928, 1,1-Ethanediol. PubChem. Retrieved [date], from https://pubchem.ncbi.nlm.nih.gov/compound/1_1-Ethanediol
Neufeld, D. (n.d.). *Water in the diffuse interstellar medium*. European Space Agency.https://www.cosmos.esa.int/documents/12133/1135364/12-1520_NeufeldD.pdf/2c34f99c-d34e-4821-844f-4a478a4eed8a
Patra, P. K., Krol, M. C., Montzka, S. A., Arnold, T., Atlas, E. L., Lintner, B. R., Stephens, B. B., Xiang, B., Elkins, J. W., Fraser, P. J., Ghosh, A., Hintsa, E. J., Hurst, D. F., Ishijima, K., Krummel, P. B., Miller, B. R., Miyazaki, K., Moore, F. L., Mühle, J., O'Doherty, S., Prinn, R. G., Steele, L. P., Takigawa, M., Wang, H. J., Weiss, R. F., Wofsy, S. C., & Young, D. (2014). Observational evidence for interhemispheric hydroxyl-radical parity. Nature, 513(7517), 219–223. https://doi.org/10.1038/nature13767
Perrero, J., Ugliengo, P., Ceccarelli, C., & Rimola, A. (2023). Quantum mechanical modeling of the grain-surface formation of acetaldehyde on H2O
Simm, G. N., Vaucher, A. C., & Reiher, M. (2019). Exploration of reaction pathways and chemical transformation networks. The Journal of Physical Chemistry. A, 123(2), 385–399. https://doi.org/10.1021/acs.jpca.8b10007
Singh, K.K., Tandon, P., Misra, A. (2019). Formation of Acetaldehyde in the Interstellar Medium from the Reaction of Methanol and Atomic Carbon in Interstellar Water Ice. In: Singh, D., Das, S., Materny, A. (eds) Advances in Spectroscopy: Molecules to Materials. Springer Proceedings in Physics, vol 236. Springer, Singapore. https://doi.org/10.1007/978-981-15-0202-6_33
Stanton, J. F., Gauss, J., Cheng, L., Harding, M. E., Matthews, D. A., Szalay, P. G., Auer, A. A., Bartlett, R. J., Benedikt, U., Berger, C., Bernholdt, D. E., Bomble, Y. J., Christiansen, O., Engel, F., Faber, R., Heckert, M., Heun, O., Huber, C., Jagau, T.-C., ... Fox, D. J. (n.d.). CFOUR, a quantum chemical program package. http://www.cfour.de
Swalen, J. D., & Costain, C. C. (1959). Internal rotation in molecules with two internal rotors: Microwave spectrum of acetone. The Journal of Chemical Physics, 31(6), 1562–1574. https://doi.org/10.1063/1.1730653
Toraya, T., Honda, S., & Fukui, S. (1979). Fermentation of 1,2-propanediol with 1,2-ethanediol by some genera of Enterobacteriaceae, involving coenzyme B12-dependent diol dehydratase. Journal of Bacteriology, 139(1), 39–47. https://doi.org/10.1128/jb.139.1.39-47.1979
Tsai, S.-R., & Hamblin, M. R. (2017). Biological effects and medical applications of infrared radiation. Journal of Photochemistry and Photobiology. B, Biology, 170, 197–207. https://doi.org/10.1016/j.jphotobiol.2017.04.014
Vatansever, F., & Hamblin, M. R. (2012). Far infrared radiation (FIR): its biological effects and medical applications. Photonics & Lasers in Medicine, 4(4), 255–266. https://doi.org/10.1515/plm-2012-0034
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Lafia Journal of Scientific and Industrial Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.









