Redox Kinetics and Mechanism of the Reactions of µ –Oxobis [aquobis (2,2’ – Bipyridine)] diruthenium (III) Ion and some Aliphatic Alcohols in Acidic Medium
DOI:
https://doi.org/10.62050/ljsir2024.v2n2.289Keywords:
dielectric constant, free radicals, ionic strength, rate constant, stoichiometryAbstract
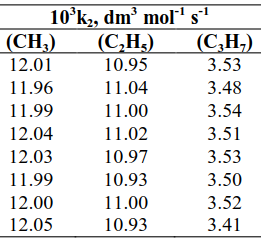
The redox kinetics and mechanisms of the reactions of μ – oxobis[aquobis(2,2’ - bipyridine)]diruthenium (III) ion, [(bipy)2(H2O)RuORu(H2O)(bipy)2]4+ (hereafter denoted as Ru2O4+ or [(H2O)2(bipy)4Ru2O]4+) and some primary aliphatic alcohols was studied in perchloric acid medium, [H+] = 5.0 x 10-3 mol dm-3, ionic strength (I), = 0.5 mol dm–3 (NaClO4), temperature (T) = 31 ± 1°C and wavelength ,λmax = 660 nm. The reactions, which led to the formation of corresponding aldehydes had a stoichiometry of 1:1, were all first order with respect to each reactant, and second order overall. The reactions proceeded in the absence of acid but when acid was added, the reactions ceased. Varying the ionic strength and dielectric constants of the reaction medium had no effect on the reaction. Added ions catalysed the reaction and free radicals were identified in the reaction mixtures in the course of the reactions. There was no evidence for the formation if intermediate complex in the course of the reaction. The order of reactivity is of the order CH3OH > C2H5OH > C3H7OH Based on the results, it is suggested that all the reactions proceeded through the outer – sphere electron transfer mechanism and a plausible mechanism that represented all the reactions is proposed.
Downloads
References
Lebeau, E.L. and Meyer, T.J. (1999). Oxidation of Benzyl Alcohol by a Dioxo Complex of Ruthenium(VI). Inorg. Chem. 38: 2174-2181, https://doi.org/10.1021/ic981040v
Catalano, V. J., Heck, R. A., Ohman, A. and Hill, M,G. (2000). Synthesis,
Characterization and Electrocatalytic Oxidation of Benzyl Alcohol by a Pair of Geometric Isomers of [Ru(trpy)(4, 4’- Me2dppi(OH2)]2+ where 4,4’- Dppi is 3,6-Di-(4-Methylpyrid-2-Yl)Pyridazine. Polyhedron 19: 1049-1055. https://doi.org/10.1016/S0277-5387(00)00347-8
Rodriguez, M, Romero, I and Llobet, A. (200). Synthesis, Structure and Redox and Catalytic Properties of a New Family of Ruthenium Complexes Containing the Tridentate bpea Ligand. Inorg. Chem., 49: 4150-4156. https://doi.org/10.1021/ic010064q
Geneste, F. and Moinet, C. (2004). Electrocatalytic Activity of Polypyridyl Ruthenium Oxo Complex Covalently Attached to a Graphite Felt Electrode. New J. Chem, 28: 722-726. https://doi.org/10.1039/B316162E
Iyun, J.F. and Shehu, A.R. (2004). Kinetics and Mechanism of the Oxidation of Ethanol and Propanol by Chromium(VI) in Acidic Medium. ChemClass Journal, 55 – 58.
Edwards, J.O. 1954. Rates of Substitution Reactions in Oxyanions. J. Chem. Educ. 31: 270. https://doi.org/10.1021/ed031p270
Gaswick, D.C. and Krueger J.H. (1969). Kinetics and Mechanism of the Chromium (VI) – Iodide Reaction. J. Am. Chem. Soc., 91: 2240. https://doi.org/10.1021/ja0103a010
Saraswat, S, Sharma, V, and Banerji, K.K. (2003). Kinetics and Mechanism of Oxidation of Aliphatic Alcohol by Quinolinium Bromochromate. Proc. Indian Acad. Sci. (Chemical. Science). 115(1): 654 – 663. https://doi.org/10.1007/BF02899321
Mathur, D., Sharma, P.K. and Banerji, K.K. (1993). Kinetics and Mechanism of the Oxidation of Primary Alcohols by Pyridinium Hydrobromide Perbromide. J. Chem. Soc., Perkins Trans., 2: 205 – 208. https://doi.org/10.1039/P29930000205
Rao, P.M, Sethuram, B. and Rao N.T. (1989). Kinetics and Mechanism of Rh(III) Catalysed Oxidation of Alcohols by Periodate. Proc. Indian Acad. Sci. 55A(6): 858 – 863.
Jerry, M. (1977). Advanced Organic Chemistry. 3rd Ed. McGraw-Hill Book Company, London. pp. 530 – 536.
Kothari, A., Kothari, S. and Banerji, K.K. (2005). Kinetics and Mechanism of the Oxidation of Alcohols by Butyltriphenylphosphonium Dichromate. Indian J. Chem., 44A: 2039 – 2043.
Aparna, P., Kothari, S. and Baneji, K.K. (1995). Kinetics and Mechanism of the Oxidation of Primary Aliphatic Alcohols by Pyridinium Bromochromate. Proc. Indian Acad. Sci. (Chemical Scienc.).107(3): 213 – 220. https://doi.org/10.1007/BF02884439
Weaver, T.R., Meyer, T.J., Adeyemi, S.A., Brown, G.M., Ecberg, R.P., Hatfield, W.E., Johnson, E.C., Murray, R.W. and Untereker, D. (1975). Chemically Significant Interactions Between Ruthenium Ions in Oxo-Bridged Complexes of Ruthenium(III). J. Am. Chem. Soc., 97: 3039-3047. https://doi.org/10.1021/ja00844a020
Vogel, A.I. (1978). Vogels’s Textbook of Quantitative Inorganic Analysis: Including Elementary Instrumental Analysis. ELBS and Longman, London. pp 125 – 247.
Iyun, J.F. and Adegite, A. (1990). Kinetics and Mechanism of the Reduction of Dichlorotetrakis(2, 2’-Bipyridine)-µ-oxodiruthenium ion by Ti(III)-EDTA in Aqueous Acidic Medium. Bull. Chem. Soc. Ethiop., 4:27-31
Vaidya, V.K., Pitlia, R.L., Kabra, B.V., Mali, S.L. and Ameta, S.C. (1991). Dye – Sensitized Photo-oxidation of Thiourea by Singlet Oxygen. Journal of Photochem. Photobiol. A 60(1): 47 – 50. https://doi.org/10.1016/1010-6030(91)90004-D
Iyun, J. F., Ayoko, G. A. and Lawal, H.M. (1992). The Stoichiometry and Kinetics of Oxidation of 1,4–Benzenediol by Diaquotetrakis(2, 2’–Bipyridine)-µ-oxodiruthenium(III) Cation in Perchloric Media. Indian J. Chem., 31(A): 943 – 947. https://doi.org/10.1016/S0277-5387(00)83529-9
Iyun, J. F., Ayoko, G. A. and Lohdip, Y. N. (1992). The Kinetics and Mechanism of the Oxidation of Diaquotetrakis(2, 2’–Bipyridine)-µ-oxodiruthenium(III) by Bromate in Aqueous Perchloric Acid. Polyhedron, 11(18): 2277-2433.
Iyun, J. F., Ayoko, G. A. and Lawal, H.M. (1992). Kinetics and Mechanism of the Oxidation of Iodide by Diaquotetrakis(2,2I–Bipyridine)-µ-oxodiruthenium(III) Ion in Acid Medium. Transit. Met. Chem., 17(1): 63 – 65.
Iyun, J. F., Ayoko., G. A. and Lohdip, Y. N. (1992). The Oxidation of Sulphite by Diaquotetrakis(2, 2’–Bipyridine)-µ-oxodiruthenium(III) Ion in Perchloric Acid. Bull. Chem. Soc. Ethiop., 6(1): 1–9.
Iyun, J. F., Ayoko, G. A. and Lawal, H.M. (1995). The Kinetics and Mechanism of the Reduction of Diaquotetrakis(2, 2’–Bipyridine)-µ-oxodiruthenium(III) by Ascorbic Acid. Transit. Met. Chem., 20(1): 30 – 33.
Iyun, J. F., Musa, K.Y. and Ayoko, G.A. (1995). Oxidation of 2 – mercaptoethanol and 2 – mercaptoethylamine by [(bpy)2H2O]RuIII]2O4+ in Aqueous Media. Indian J. Chem., 34(A): 635 – 638.
Iyun, J. F, Ayoko, G.A and Lawal, H.M. (1996). Kinetics of the Reduction of µ-oxobis [aquobis(2, 2’- bipyridine)] Ruthenium(III) by l–cysteine in Aqueous Solution. Indian J. Chem., 35(A): 210 – 213. https://doi.org/10.1007/BF03325418
Zaidi, S.A.H. (1991). The Influence of Dielectric Constant Variations on the Kinetics of Reaction Between Bromate and Tellurite Ions in Aqueous Ethanol Mixed Solvents. J. Chem. Soc. Pak., 13(2): 67-70
Vogel, A.I. (1958). Qualitative Organic Analysis. 2nd Ed. Longman, London p117.
Lee, D.G. and Congson, L.N. (1990). Kinetics and Mechanisms of the Oxidation of Alcohols by Ruthenate and Perruthenate. Can. J. Chem. 68 (1): 1774 – 1779.
Harit, H., Hiran, B. L. and Joshi, S.N. (2015). Kinetics and Mechanism of Oxidation of Primary Alcohols by Pyridinium Dichromate. Chem. Sci. Trans., 4(1): 49 – 58.
Mittal, S, Sharma, V. and Banerji, K.K. (2004). Kinetics and Mechanism of the Oxidation of Primary Alcohols by Sodium N – Chloroethylcarbamate. Int. J. Chem. Kinet.. 18(6): 689 – 699.
Lee, D.G. and Spitzer, U.A. (1975). Kinetics and Mechanisms of the Oxidation of Benzyl Alcohol and Benzaldehyde by Aqueous Sodium Dichromate. Can. J. Chem., 53: 3709-3712
Bijudas, K. (2014). Kinetics and Mechanisms of the Selective Oxidation of Benzyl alcohols by Acidified Dichromate in Aqueous Acetic Acid. Orient. J. Chem., 30(3): 45 – 49.
Sengupta, K.K, Samanta, T. and Basu, S.N. (1986). Kinetics and Mechanism of Oxidation of Ethanol, Isopropanol and Benzyl Alcohol by Chromium(VI) in Perchloric Acid Medium. Tetrahedron, 42(2): 681 – 685.
Dhage, S.D., Patwari, S.B. and Mukhedkar, S. (2013). Kinetics and Mechanisms of Oxidation of Benzyl Alcohol and Cyclohexanol by Quinolinium Fluorochromate. J. Chem. Pharm. Res. 5(5): 41 – 45.
Dharmaraja, J., Krishnasamy, K. & Shanmugam, M. (2008). Kinetics and mechanism of oxidation of benzyl alcohol by benzimidazolium fluorochromate. E - J. Chem., 5(4): 754 – 760. http://dx.doi.org/10.1155/2008/426508
Mukherjee, J. & Banerji, K. K. (1980). Kinetics and mechanism of the oxidation of substituted benzyl alcohols by sodium N–chlorobenzenesulfonamide. J. Chem. Soc., Perkin Trans., 1324-1327. https://doi.org/10.1039/P29800000676
Kothari, S. & Banerji, K. K. (2011). Kinetics and mechanism of the oxidation of substituted benzyl alcohols by sodium N-bromobenzenesulphonamide. Can. J. Chem., 63(10), 2726 – 2729. https://doi.org/10.1139/v85-452
Hiremath, R. C., Jagadeesh, R. V., Puttaswamy, C. & Mayana, S. (2005). Kinetics and mechanisms of oxidation of chloramphenicol by 1–chlorobenzotriazole in acidic medium. J. Chem. Soc., 117(4), 333–336. http://dx.doi.org/10.1007/BF02708447
Nimbalkar, L. V. & Chavan, A. M. (1998). Kinetics and Mechanism of cerium (III) oxidation of primary alcohols by chromium (III). J. Phys. Org. Chem., 11(10), 697 – 700. https://doi.org/10.1002/(SICI)1099-1395
Atkins, P.W. 1979. Physical Chemistry. English Language Book Society and Oxford University Press. Oxford, pp. 914–917
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Lafia Journal of Scientific and Industrial Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.









