Determination of Antibiotic-Resistant Genes in Bacteria from Borehole Water Samples in Kaduna Metropolis
DOI:
https://doi.org/10.62050/ljsir2026.v4n1.602Keywords:
Antimicrobial resistance, Polymerase chain reaction, Multiple resistant determinants, GeneAbstract
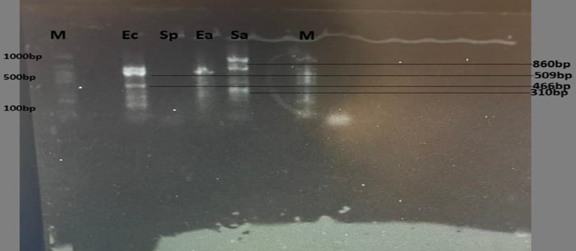
The persistence of drug-resistant microorganisms in environmental water sources, particularly boreholes, presents growing challenges for public health, especially within densely populated urban locales. In Kaduna Metropolis, borehole water serves as a crucial means of potable water supply; however, the infiltration of bacteria with antibiotic resistance into these sources represents a concerning vector for advancing antimicrobial resistance (AMR). This investigation explored the genetic mechanisms of bacterial strains retrieved from borehole water across four administrative districts Chikun, Igabi, Kaduna North, and Kaduna South spanning dry and wet climatic periods. A cumulative total of 200 samples were acquired and subjected to bacteriological assessment using established culture-based methodologies. Polymerase Chain Reaction (PCR) techniques were utilized to screen for specific resistance-related genes, including blaTEM, gyrA, sul1, and PBP2a. Molecular screening identified multiple resistance determinants, with sul1 being the most frequently encountered. These findings point to the role of borehole water systems in harboring resistant microbial populations and underline environmental contributions to AMR transmission. The identified geographical and seasonal disparities in bacterial occurrence further emphasize the impact of regional infrastructure and environmental dynamics, reinforcing the call for sustained monitoring and proactive public health strategies, even in the absence of seasonal or locational trends in resistance behavior.
Downloads
References
Cabral, J. P. (2010). Water microbiology: Bacterial pathogens and water. International Journal of Environmental Research and Public Health, 7(10), 3657-703.
United Nations International Children’s Emergency Fund (UNICEF). (2024). Water, sanitation, and hygiene (WASH) in communities: Impact on global health. Retrieved from https://www.unicef.org
United Nations. (UN). (2019). About the sustainable development goals [Internet]. Available from https://www.un.org/sustainable development /sustainable-development-goals/
World Health Organization (WHO). (2017). Guidelines for drinking water quality: fourth edition incorporating the first addendum. Geneva.
Bessong, P. O., Odiyo, J. O., Musekene, J. N., and Tessema, A. (2009). Spatial Distribution of Diarrhoea and Microbial Quality of Domestic Water during an Outbreak of Diarrhoea in the Tshikuwi Community in Venda, South Africa. Journal of Health, Population and Nutrition, 27(6), 652-659.
Bockelmann, U., Dorries, H. H., Ayuso-Gabella, M. N., Salgot de Marçay, M., Tandoi, V., Levantesi, C., and Wintgens, T. (2009). Quantitative PCR monitoring of antibiotic resistance genes and bacterial pathogens in three European artificial groundwater recharge systems. Applied and Environmental Microbiology, 75(1), 154-163.
Ogenyi F. C. (2023). An Appraisal of Waterborne Diseases in Nigeria: Focus on Effective Management. International Digital Organization for Scientific Research (IDOSR) Journal of Experimental Sciences, 9(1) 5-10.
National Population Commission (NPC) & Labour and Community Foundation (LCF). (2014). Kaduna state population and infrastructure baseline report. Abuja, Nigeria: NPC and LCF
Google search engine https://www.google.com/maps/search/Map+indicating+borehole+water+sampling+zones+in+Kaduna+metropolis/@10.5059616,7.4306571,13z?entry=ttu&g_ep=EgoyMDI1MDYwMy4wIKXMDSoASAFQAw%3D%3D
Hassan A. U., A. H. Madu, U. O. Ozojiofor, A. H. Galadanci, I. B. Mato and R. Jafaru (2021), Antimicrobial Activities of Cymbopogon citratus and Ximenia Americana Leaf Extracts Against Some Selected Bacterial and Yeast Clinical Isolates. Asian Journal of Biochemistry, Genetics and Molecular Biology; 9(1): 1-10, DOI: 10.9734/AJBGMB/2021/v9i130204
Cheesbrough, M. (2006). District Laboratory Practice in Tropical Countries (Part 2). Cambridge University Press.
Koneman, E. W., Allen, S. D., Janda, W. M., Schreckenberger, P. C., & Winn, W. C. (2016). Koneman's Color Atlas and Textbook of Diagnostic Microbiology (7th ed.). Lippincott Williams & Wilkins.
Hassan AU, Garba S, SB Ayuba Buhari, IB Mato, AH Madu, Aminu A, UO Ozojiofor and Egbe NE (2023) Antibacterial activity of five selected ethno-medicinal plants against extreme multidrug resistance strains of Pseudomonas aeruginosa and Staphylococcus aureus. Journal of Current Biomedical Science, Vol 3(6), 1364- 1396.
American Public Health Association. (2017). Standard methods for the examination of water and wastewater (23rd ed.). American Public Health Association.
Zaky, M. M., and Ibrahim, M. E. (2017). Screening of bacterial and fungal biota associated with Oreochromis niloticus in Lake Manzala and its impact on human health. Health, 9(4), 697-71
Hassan AU, Garba S, Sherifat Ayuba-Buhari, Appah J, UO Ozojiofor and Egbe NE (2023) Antibacterial activity of Acacia nilotica and Jatropha curcas plants leave extracts against multi-drug resistant strains of Pseudomonas aeruginosa and Staphylococcus aureus. International Journal of Medical and All Body Health Research, 4 (3), 34-46
Innis, M. A., & Gelfand, D. H. (1990). PCR Protocols: A Guide to Methods and Applications. Academic Press.
Clarridge, J. E. (2004). Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clinical Microbiology Reviews, 17(4), 840-862.
Rehm, H. L., Bale, S. J., Bayrak-Toydemir, P., Berg, J. S., Brown, K. K., and Deignan, J. L. Working Group of the American College of Medical Genetics and Genomics Laboratory Quality Assurance Committee. (2013). ACMG clinical laboratory standards for next-generation sequencing. Genetics in Medicine, 15(9), 733–747.
Adekanmbi, A. O., and Falodun, O. I. (2018). Detection of blaTEM resistance gene in bacteria described as Staphylococcus aureus isolated from surgical site infections. Global Journal of Science Frontier Research: C Biological Science, 18(2), 25–30.
Patoli, A. A., Wazir, S., & Khan, A. A. (2010). High prevalence of multi drug-resistant Escherichia coli in drinking water samples from Hyderabad. Gomal Journal of Medical Sciences, 8(1), 23–26.
Agyekum, S. T. (2022). Antibiotic-resistant bacteria and resistance genes in isolates from Ghanaian drinking water sources. Environmental Science and Pollution Research, 29(34), 51012–51022.
Orji, J. C., Onwuezobe, I. A., and Mbata, T. I. (2016). Prevalence, antibiogram and molecular characterization of community-acquired methicillin-resistant Staphylococcus aureus in Awka, Anambra, Nigeria. Journal of Infection in Developing Countries, 10(2), 146–153.
Ohaegbulem, A. S., Akinnibosun, O., and Akuakolam, I. M. (2023). Detection of PBP2a and PVL genes among Staphylococcus aureus and their methicillin-resistant strains isolated from a hospital in Sokoto Town. Microbes and Infectious Diseases, 4(4), 1210–1218.
Downloads
Published
Issue
Section
License
Copyright (c) 2026 Precious Opeyemi Oghenetega , Nkechi Eucharia Egbe, Jeremiah Appah, Chukwudi Kingsley Onuh , Abba Umar Hassan (Author)

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.









