Resistance to Azole Drugs by Fungal Species Isolated from Date Palm Samples within Lafia Metropolis, Nigeria
DOI:
https://doi.org/10.62050/ljsir2024.v2n2.306Keywords:
Fruits, Azole, Aspergillus, Contamination, ResistanceAbstract
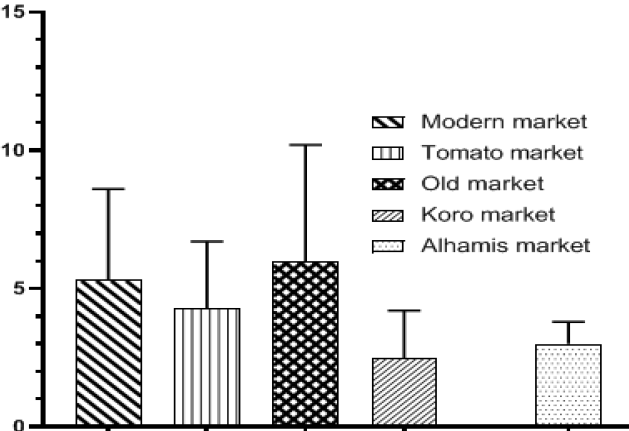
Fungal species infecting fruits are developing resistance to some antifungals. The study determined prevalent fungi in date palm fruits and their resistance to azole antifungals. Dried date palm fruit samples were collected from Modern Market, Old Market, Alhamis Market, Koro Market and Tomato Market in Lafia Metropolis, Nigeria and fungi isolated from them. Antifungal susceptibility test as a zone of inhibition of fungal mycelial growth were recorded for terbinafine, clotrimazole, nystatin, metronidazole and fluconazole. Dates from the old market had the highest contamination with 6.0×103 ± 4.2 cfu/g, while samples from Koro market with 2.5×103 ± 1.7 cfu/g were the least contaminated. A total of 84 fungi were isolated, Aspergillus niger was the most predominant species while Aspergillus versicolor was the least predominant. Terbinafine was the most effective azole against the fungal species isolated with a zone of inhibition of 43 mm in diameter, while fluconazole was the least effective with the zone of inhibition of 0 mm. The organism most susceptible to the antifungal drugs was Aspergillus niger, while Penicillium chrysogenum was the most resistant. Results of this study indicated that, date palm fruits around the Lafia markets were more susceptible to Aspergillus niger contamination than other fungal species, and Terbinafine was the best azole antifungal drug. The study recommends that further research should be carried out with respect to the resistance developed against azoles by the fungal species.
Downloads
References
Ling, L., Luo, H., Zhao, Y., Yang, C., Cheng, W., & Pang, M. (2023). Fungal pathogens causing postharvest fruit rot of wolfberry and inhibitory effect of 2,3-butanedione. Frontiers in Microbiology, 13, 1068144. https://doi.org/10.3389/fmicb.2022.1068144
Alegbeleye, O. O., Singleton, I., & Sant'Ana, A. S. (2018). Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiology, 73, 177–208. https://doi.org/10.1016/j.fm.2018.01.003
Imade, F., Ankwasa, E. M., Geng, H., Ullah, S., Ahmad, T., Wang, G., Zhang, C., Dada, O., Xing, F., Zheng Y, Liu Y. (2021). Updates on food and feed mycotoxin contamination and safety in Africa with special reference to Nigeria. Mycology, 20,12(4):245-260. https://doi.org/10.1080/21501203.2021.1941371.
Ekwomadu, T. I., Akinola, S. A., & Mwanza, M. (2021). Fusarium Mycotoxins, Their Metabolites (Free, Emerging, and Masked), Food Safety Concerns, and Health Impacts. International Journal of Environmental Research and Public Health, 18(22), 11741. https://doi.org/10.3390/ijerph182211741
Ahmad Mohd Zain, M. R., Abdul Kari, Z., Dawood, M. A. O. et al. Bioactivity and Pharmacological Potential of Date Palm (Phoenix dactylifera L.) Against Pandemic COVID-19: a Comprehensive Review. Applied Biochemistry and Biotechnology, 194, 4587–4624 (2022). https://doi.org/10.1007/s12010-022-03952-2
Al-Shwyeh H. A. (2019). Date Palm (Phoenix dactylifera L.) Fruit as Potential Antioxidant and Antimicrobial Agents. Journal of Pharmacy & Bioallied Sciences, 11(1), 1–11. https://doi.org/10.4103/jpbs.JPBS_168_18
Rosam, K., Monk, B. C., & Lackner, M. (2021). Sterol 14α-Demethylase Ligand-Binding Pocket-Mediated Acquired and Intrinsic Azole Resistance in Fungal Pathogens. Journal of Fungi 2021, 7, 1. https://doi.org/10.3390/jof7010001
Emami, S., Tavangar, P., & Keighobadi, M. (2017). An overview of azoles targeting sterol 14α-demethylase for antileishmanial therapy. European Journal of Medical Chemistry, 135, 241-259. https://doi.org/10.1016/j.ejmech.2017.04.044.
Herrick, E. J., & Hashmi, M. F. (2023). Antifungal Ergosterol Synthesis Inhibitors. [Updated 2023 Feb 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551581/
Ivanov, M., Ćirić, A., & Stojković, D. (2022). Emerging Antifungal Targets and Strategies. International. Journal Molecular Science, 23, 2756. https://doi.org/10.3390/ijms23052756
Dayan, M. P. (2004). Fungal Diseases of forest tree seeds and control measures: A Guidebook. DENR Recommends, 13: 1-25.
Cheesebrough, M. (2000). District Laboratory Practice in Tropical Countries Part 2, Cambridge University Press, Cambridge. 47-54.
Orole, O. O., Okolo, I. O., Fadayomi V., Odonye, D. I., & Ohiobo, A. (2017). Fungal Species Associated with Date Palm (Phoenix dactylifera L.) Fruit and Tiger Nut (Cyperus esculentus L.) Fruit in Lafia Metropolis, Nasarawa State, Nigeria. Archives of Current Research International, 9(4), 1-7. https://doi.org/10.9734/ACRI/2017/36527
Udeozor, L. O., & Awonorin, S. O. (2014). Comparative microbial analysis and storage of tiger nut soy milk extract. Australian Journal of Nutritional Food Science, 2(5), 1-6.
Agbaje, R. B., Oyetayo, V. O., & Ojokoh, A. O. (2015). Assessment of the microbial and physicochemical composition of tiger nut subjected to different fermentation methods. Pakistan Journal of Nutrition, 14(11), 742-748.
Garaiová, M., Zambojová, V., Šimová, Z., Griač, P., & Hapala, I. (2014). Squalene epoxidase as a target for manipulation of squalene levels in the yeast Saccharomyces cerevisiae. FEMS Yeast Research, 14(2): 310–323. https://doi.org/10.1111/1567-1364.12107
Draskau, M. K., & Svingen, T. (2022). Azole Fungicides and Their Endocrine Disrupting Properties: Perspectives on Sex Hormone-Dependent Reproductive Development. Frontiers in Toxicology, 4, 883254. https://doi.org/10.3389/ftox.2022.883254
Sen, P., Vijay, M., Singh, S., Hameed, S., & Vijayaraghavan, P. (2022). Understanding the environmental drivers of clinical azole resistance in Aspergillus species. Drug target insights, 16, 25–35. https://doi.org/10.33393/dti.2022.2476
Fisher, M. C., Alastruey-Izquierdo, A., Berman, J. et al. (2022). Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiology, 20, 557–571. https://doi.org/10.1038/s41579-022-00720-1
Lopez-Ribot, J. L. McAtee, R. K., & Lee, L.N. (1998). Distinct patterns of gene expression associated with the development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrobial Agents and Chemotherapy, 42:2932–7.
Bhattacharya, S., Sae-Tia, S., & Fries, B. C. (2020). Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics (Basel, Switzerland), 9(6), 312. https://doi.org/10.3390/antibiotics9060312
Zavrel, M., Esquivel, B.D., & White, T.C. (2014). The Ins and Outs of Azole Antifungal Drug Resistance: Molecular Mechanisms of Transport. In: Gotte, M., Berghuis, A., Matlashewski, G., Wainberg, M., Sheppard, D. (eds) Handbook of Antimicrobial Resistance. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0667-3_29-1
Patil, S., Majumdar, B., Sarode, S. C., Sarode, G. S., & Awan, K. H. (2018). Oropharyngeal Candidosis in HIV-Infected Patients-An Update. Frontiers in Microbiology, 9, 980. https://doi.org/10.3389/fmicb.2018.00980
Logan, A., Wolfe, A., & Williamson, J. C. (2022). Antifungal Resistance and the Role of New Therapeutic Agents. Current Infectious Disease Reports, 24(9), 105–116. https://doi.org/10.1007/s11908-022-00782-5
Shishodia, S. K., Tiwari, S., & Shankar, J. (2019). Resistance mechanism and proteins in Aspergillus species against antifungal agents, Mycology, 10(3), 151-165, https://doi.org/10.1080/21501203.2019.1574927
Gonzalez-Jimenez, I., Lucio, J., Amich, J., Cuesta, I., Sanchez Arroyo, R., Alcazar-Fuoli, L., & Mellado, E. (2020). A Cyp51B Mutation Contributes to Azole Resistance in Aspergillus fumigatus. Journal of Fungi, 6, 315. https://doi.org/10.3390/jof6040315
Lucio, J., Gonzalez-Jimenez, I., Rivero-Menendez, O., Alastruey-Izquierdo, A., Pelaez, T., Alcazar-Fuoli, L., & Mellado, E. (2020). Point Mutations in the 14-α Sterol Demethylase Cyp51A or Cyp51C Could Contribute to Azole Resistance in Aspergillus flavus. Genes, 11, 1217. https://doi.org/10.3390/genes11101217
Mellado, E., Garcia-Effron, G., Alcázar-Fuoli, L., Melchers, W. J., Verweij, P. E., Cuenca-Estrella, M., & Rodríguez-Tudela, J. L. (2007). A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrobial agents and chemotherapy, 51(6), 1897–1904. https://doi.org/10.1128/AAC.01092-06
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Lafia Journal of Scientific and Industrial Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.









