KINETICS OF THE OXIDATION OF ORANGE II BY NITRITE ION IN AQUEOUS ACIDIC MEDIUM
Mots-clés :
Kinetics, Nitrite, Mechanism, Orange IIRésumé
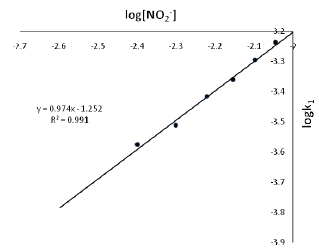
The kinetics of the electron transfer reaction between Orange II(here and thereafter referred to as OII-) and nitrite ion in aqueous acidic solution has been studied spectrophotometrically (λ = 484 nm) at T = 27 ± 1oCin the acid range 1.0 × 10-2 ≤ [H+] ≤ 10.0 × 10-2 mol dm-3, ionic strength 0.1 ≤ [I] ≤ 0.7 mol dm-3 (NaCl). The reaction shows a first order dependence on [oxidant] and [reductant].The rate of the reaction increases with increase in [H+]. Plot of k2versus [H+] was linear with an intercept. The overall reaction conforms to the rate law: -d[OII-]/dt = (a + b[H+])[OII-][NO2-] The stoichiometry of the reaction is 1:2 (OII- : NO2-). Added cations and anions speed up the rate of the reaction. The results of spectroscopic investigation indicate that no intermediate complex is probably formed
in the course of this reaction. The reaction is believed to proceed via the outersphere mechanistic pathway.
##plugins.themes.default.displayStats.downloads##